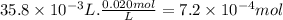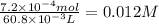Chemistry, 13.11.2019 23:31 jetskiinbunny31
Glycolic acid, which is a monoprotic acid and a constituent in sugar cane, has a pka of 3.9. a 25.0 ml solution of glycolic acid is titrated to the equivalence point with 35.8 ml of 0.020 m sodium hydroxide solution. what is the ph of the resulting solution at the equivalence point?

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 02:40
Consider the nuclear equation below. 239/94 pu—-> x+ 4/2 he. what is x?
Answers: 2

Chemistry, 22.06.2019 04:30
Which is a chemical property of iron? a. it forms iron oxide (rust) when exposed to moisture and air. b. it is a gray–black metal that is hard to the touch. c. it has a melting point of 2795°f (1536°c). d. it is a good conductor of heat
Answers: 2

Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1
You know the right answer?
Glycolic acid, which is a monoprotic acid and a constituent in sugar cane, has a pka of 3.9. a 25.0...
Questions

Chemistry, 01.09.2020 23:01

Mathematics, 01.09.2020 23:01


Mathematics, 01.09.2020 23:01


Chemistry, 01.09.2020 23:01


Biology, 01.09.2020 23:01

English, 01.09.2020 23:01


Mathematics, 01.09.2020 23:01



Business, 01.09.2020 23:01

English, 01.09.2020 23:01

History, 01.09.2020 23:01

Business, 01.09.2020 23:01

Mathematics, 01.09.2020 23:01


Mathematics, 01.09.2020 23:01