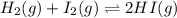H2 and i2 react in an exothermic reaction according to the following equation:
h2(g) + i2(g) double arrow yields 2hi (g)
h2 and i2 are placed in a sealed container and are allowed to reach equilibrium. what could you change about the system that would shift its equilibrium position?
a. add a catalyst.
b. decrease the volume.
c. increase the pressure.
d. lower the temperature.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 09:30
What are scientists who study fossils called? ( a ) astronomers. ( b ) biologists. ( c ) geologists. ( d ) paleontologists.
Answers: 2

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

You know the right answer?
H2 and i2 react in an exothermic reaction according to the following equation:
h2(g) + i2(g)...
h2(g) + i2(g)...
Questions


Chemistry, 23.04.2021 15:20



Business, 23.04.2021 15:20

Mathematics, 23.04.2021 15:20

Computers and Technology, 23.04.2021 15:20

Health, 23.04.2021 15:20

Mathematics, 23.04.2021 15:20

Mathematics, 23.04.2021 15:20







History, 23.04.2021 15:20

Mathematics, 23.04.2021 15:20

Mathematics, 23.04.2021 15:20