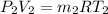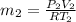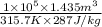Chemistry, 14.11.2019 02:31 jimmymurray29
Arigid, well-insulated tank is filled initially with 5.0 kg of air at a pressure of 5 bars and a temperature of 500k. a leak develops, and air slowly escapes until the pressure of the air remaining in the tank is 1 bar. employing the ideal gas model, determine the amount of mass remaining in the tank and its temperature.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:50
The density of glycerin is 1.26grams/centimeter cubed . how many is this? use the conversion rates of and . express your answer to the correct number of significant figures.
Answers: 1

Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2

Chemistry, 22.06.2019 21:00
Read "who built the pyramids? ”. leave this link open while you answer the questions throughout the assignment. give at least two reasons why some people claim the pyramids of giza were constructed by aliens.
Answers: 1

Chemistry, 23.06.2019 02:00
Calculate the molarity of each aqueous solution: a. 78.0 ml of 0.240 m naoh diluted to 0.250 l with water b. 38.5 ml of 1.2 m hno3 diluted to 0.130 l with water
Answers: 1
You know the right answer?
Arigid, well-insulated tank is filled initially with 5.0 kg of air at a pressure of 5 bars and a tem...
Questions

Biology, 28.01.2020 03:31


English, 28.01.2020 03:31

English, 28.01.2020 03:31

English, 28.01.2020 03:31

Mathematics, 28.01.2020 03:31

History, 28.01.2020 03:31



Mathematics, 28.01.2020 03:31



Biology, 28.01.2020 03:31

Mathematics, 28.01.2020 03:31

English, 28.01.2020 03:31

History, 28.01.2020 03:31


Mathematics, 28.01.2020 03:31


Mathematics, 28.01.2020 03:31

![\frac{T_{2}}{T_{1}} = [\frac{P_{2}}{P_{1}}]^{\frac{k - 1}{k}}](/tpl/images/0373/3813/e918f.png)
![T_{2} = T_{1} \times [\frac{P_{2}}{P_{1}}]^{\frac{k - 1}{k}}](/tpl/images/0373/3813/79df8.png)
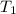 = 500 K, m = 5 kg
= 500 K, m = 5 kg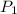 = 5 bar,
= 5 bar, 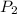 = 1 bar
= 1 bar![T_{2} = 500 K \times [\frac{5 bar}{1 bar}]^{\frac{1.4 - 1}{1.4}}](/tpl/images/0373/3813/b6551.png)
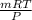
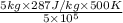 (as 1 bar = 10^{5} Pa[/tex])
(as 1 bar = 10^{5} Pa[/tex])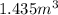
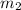 as follows.
as follows.