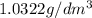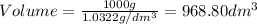1. for dry air at 1. atm pressure, the densities at –50°c, 0°c, and 69°c are 1.5826 g dm–3 , 1.2929 g dm–3, and 1.0322 g dm–3, respectively. a) assume a sample of mass 1000 g, and calculate the volume at each temperature. b) from these data, and assuming that air obeys charles’s law, determine a value for th

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2


Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1

Chemistry, 23.06.2019 08:00
Technician a says that you should never jump-start a frozen battery. technician b says that a frozen battery can explode, causing injury, when jump-started. who is correct?
Answers: 2
You know the right answer?
1. for dry air at 1. atm pressure, the densities at –50°c, 0°c, and 69°c are 1.5826 g dm–3 , 1.2929...
Questions


Mathematics, 29.09.2019 18:30

Mathematics, 29.09.2019 18:30


English, 29.09.2019 18:30

Mathematics, 29.09.2019 18:30

Mathematics, 29.09.2019 18:30


History, 29.09.2019 18:30

Mathematics, 29.09.2019 18:30

Arts, 29.09.2019 18:30

Mathematics, 29.09.2019 18:30

Health, 29.09.2019 18:30


Social Studies, 29.09.2019 18:30

Mathematics, 29.09.2019 18:30

Biology, 29.09.2019 18:30



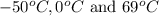 are
are 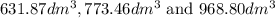 respectively.
respectively.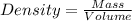
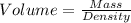
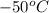 :
: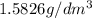
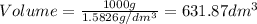
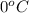 :
: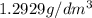
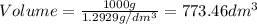
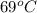 :
: