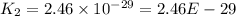Chemistry, 14.11.2019 06:31 strawberrymochi390
The reaction 2no2 → 2no + o2 obeys the rate law: rate = 1.4 x 10-2[no2]2 at 500 k . what would be the rate constant at 119 k if the activation energy is 80. kj/mol? this is a second order reaction, giving k the units of m-1s-1 this will not change with the change in temperature. do not include units in your answer. exponential numbers need to be entered like this: 2 e-1 means 2 x 10-1. the rate constant, k, at 119 k equals:

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
14. complete and balance the equations for the single displacement reactions. a. zn + pb(no3)2 -> b. al + niso4 -> 15. complete and balance the equations for the double displacement reactions. a. agno3(aq) + nacl(aq) -> b. mg(no3)2(aq) + koh(aq) -> 16. complete and balance the equations for the combustion reactions. a. __ ch4 + o2 -> b. __ c3h6 + o2 -> c. + o2 ->
Answers: 2

Chemistry, 22.06.2019 02:30
List four observations that indicate that a chemical reaction may be taking place
Answers: 1

Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1

You know the right answer?
The reaction 2no2 → 2no + o2 obeys the rate law: rate = 1.4 x 10-2[no2]2 at 500 k . what would be t...
Questions




Mathematics, 12.02.2021 20:30


Mathematics, 12.02.2021 20:30

Chemistry, 12.02.2021 20:30



Chemistry, 12.02.2021 20:30

Mathematics, 12.02.2021 20:30

Mathematics, 12.02.2021 20:30





Arts, 12.02.2021 20:30

Spanish, 12.02.2021 20:30


History, 12.02.2021 20:30

![Rate=1.4\times 10^{-2}[NO_2]^2](/tpl/images/0373/7605/5818c.png) ..........(1)
..........(1)![Rate=k[NO_2]^2](/tpl/images/0373/7605/d48ef.png) ............(2)
............(2)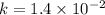
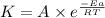
![\log (\frac{K_2}{K_1})=\frac{Ea}{2.303\times R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0373/7605/6d953.png)
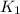 = rate constant at
= rate constant at 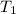 =
= 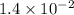
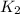 = rate constant at
= rate constant at 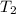 = ?
= ?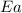 = activation energy for the reaction = 80.0 kJ/mole = 80000 J/mole
= activation energy for the reaction = 80.0 kJ/mole = 80000 J/mole![\log (\frac{K_2}{1.4\times 10^{-2}})=\frac{80000J/mole}{2.303\times 8.314J/mole.K}[\frac{1}{500}-\frac{1}{119}]](/tpl/images/0373/7605/44fe7.png)