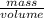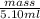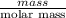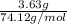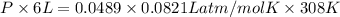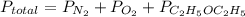Asample of 5.10 ml of diethylether (c2h5oc2h5; density = 0.7134 g/ml) is introduced into a 6.00 -l vessel that already contains a mixture of n2 and o2, whose partial pressures are pn2 = 0.752 atm and po2 = 0.206 atm. the temperature is held at 35.0 °c, and the diethylether totally evaporates.
a) calculate the partial pressure of the diethylether.
b) calculate the total pressure in the container.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:00
Look at the bean data from days 4–6. use these data to explain how natural selection changed the number of dark red walking beans over time. writing part
Answers: 3

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 23.06.2019 06:00
What physical property of gold makes panning a useful way to get gold from streams?
Answers: 2

Chemistry, 23.06.2019 11:30
Which of the following is a property of an acid solution? a. slippery to the touch b. ph less than 7 c. turns red litmus paper blue d. bitter taste
Answers: 1
You know the right answer?
Asample of 5.10 ml of diethylether (c2h5oc2h5; density = 0.7134 g/ml) is introduced into a 6.00 -l...
Questions

Mathematics, 16.04.2021 20:10

Biology, 16.04.2021 20:10

Mathematics, 16.04.2021 20:10

Spanish, 16.04.2021 20:10




Engineering, 16.04.2021 20:10




English, 16.04.2021 20:10




Mathematics, 16.04.2021 20:10

Arts, 16.04.2021 20:10


Mathematics, 16.04.2021 20:10

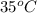 = (35 + 273) K = 308 K
= (35 + 273) K = 308 K