
Chemistry, 23.08.2019 20:40 hbkakabryce0p3fkoq
Use electron transfer or electron shift to identify what is oxidized and what is reduced in each reaction :
a) 2na(s) + br2(l) > 2nabr(s)
b) h2(g) + cl2(g) > 2hcl(g)
c) 2li(s) + f2(g) > 2lif(s)
d) s(s) + cl2(g) > scl2(g)
e)n2(g) + 2o2(g) > 2no2(g)
f) mg(s) +cu(no3)2(aq) = mg(no3)2(aq) + cu(s)
for each reaction above, identify the reducing agent and the oxidizing agent

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 11:40
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2
You know the right answer?
Use electron transfer or electron shift to identify what is oxidized and what is reduced in each rea...
Questions

Mathematics, 11.01.2021 20:30


Biology, 11.01.2021 20:30

Biology, 11.01.2021 20:30

Chemistry, 11.01.2021 20:30




History, 11.01.2021 20:30


Mathematics, 11.01.2021 20:30




Business, 11.01.2021 20:30


Mathematics, 11.01.2021 20:30

Mathematics, 11.01.2021 20:30


Mathematics, 11.01.2021 20:30




 is reduced in this reaction. The reducing agent is, 'Na' and oxidizing agent is,
is reduced in this reaction. The reducing agent is, 'Na' and oxidizing agent is, 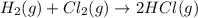
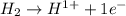

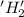 is oxidized and
is oxidized and  is reduced in this reaction. The reducing agent is,
is reduced in this reaction. The reducing agent is, 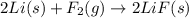


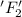 is reduced in this reaction. The reducing agent is, 'Li' and oxidizing agent is,
is reduced in this reaction. The reducing agent is, 'Li' and oxidizing agent is, 




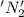 is oxidized and
is oxidized and  is reduced in this reaction. The reducing agent is,
is reduced in this reaction. The reducing agent is, 
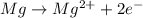

 is oxidized and
is oxidized and  is reduced in this reaction. The reducing agent is, 'Mg' and oxidizing agent is, 'Cu'.
is reduced in this reaction. The reducing agent is, 'Mg' and oxidizing agent is, 'Cu'.

