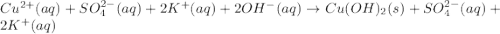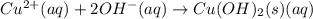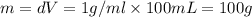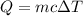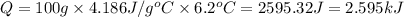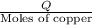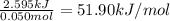Consider two solutions, the first being 50.0 ml of 1.00 m cuso4 and the second 50.0 ml of 2.00 m koh. when the two solutions are mixed in a constant-pressure calorimeter, a precipitate forms and the temperature of the mixture rises from 21.5 ∘c to 27.7 ∘c.
a) before mixing, how many grams of cu are present in the solution of cuso4?
b) predict the identity of the precipitate in the reaction.
c) write complete equation for the reaction that occurs when the two solutions are mixed.
d) write net ionic equation for the reaction that occurs when the two solutions are mixed.
e) from the calorimetric data, calculate δh for the reaction that occurs on mixing. assume that the calorimeter absorbs only a negligible quantity of heat, that the total volume of the solution is 100.0 ml, and that the specific heat and density of the solution after mixing are the same as that of pure water.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1

Chemistry, 22.06.2019 11:00
Surface currents are caused by blank space . question 14 options: surface currents are caused by? differences in water temperature high salinity differences in density wind forces
Answers: 1

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

You know the right answer?
Consider two solutions, the first being 50.0 ml of 1.00 m cuso4 and the second 50.0 ml of 2.00 m koh...
Questions

Mathematics, 22.03.2021 17:00

Mathematics, 22.03.2021 17:00

Mathematics, 22.03.2021 17:00




Mathematics, 22.03.2021 17:00



Mathematics, 22.03.2021 17:00

Mathematics, 22.03.2021 17:00








Mathematics, 22.03.2021 17:00

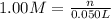
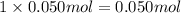 of copper
of copper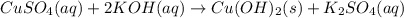
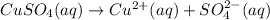 ..[1]
..[1]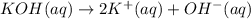 ..[2]
..[2]