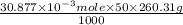Chemistry, 14.11.2019 20:31 SucMaDongShan
Using the henderson-hasselbalch equation, calculate the amount of hepes (sodium salt) and hepes (free acid) required to prepare 50 ml of a 100 mm buffer that is ph = 7.20. the pka of hepes is 7.55 at 20° c. the formula weight of the sodium salt is 260.31. the formula weight of the free acid is 238.31. weigh out the appropriate amounts of the hepes (sodium salt) and hepes (free acid), transfer to a 100 ml beaker, dissolve in deionized water to an approximate volume of 40 ml

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2

Chemistry, 23.06.2019 01:10
Volume is a measurement of how fast particles of a substance are moving
Answers: 3
You know the right answer?
Using the henderson-hasselbalch equation, calculate the amount of hepes (sodium salt) and hepes (fre...
Questions


Mathematics, 20.10.2020 18:01


Chemistry, 20.10.2020 18:01

Biology, 20.10.2020 18:01

Chemistry, 20.10.2020 18:01




Mathematics, 20.10.2020 18:01

Mathematics, 20.10.2020 18:01



History, 20.10.2020 18:01

Health, 20.10.2020 18:01

Mathematics, 20.10.2020 18:01

Biology, 20.10.2020 18:01



Mathematics, 20.10.2020 18:01

![pK_{a} + log \frac{[Salt]}{[Acid]}](/tpl/images/0374/5521/81f72.png)
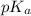 = 7.55.
= 7.55.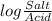
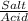 = 0.4467
= 0.4467 = 100 mM
= 100 mM 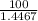
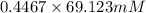
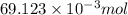 .
.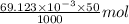
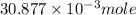 .
.