
Suppose that some fescn2+ is added to the above solution to shift the equilibrium. when equilibrium is re-established, the following concentrations are found.
a) [fe3+ ] = 8.12 ✕ 10−3 m,
b) [scn − ] = 7.84 ✕ 10−3 m
what is the concentration of fescn2+ in the new equilibrium mixture?
hint: use your value of k to solve for the unknown concentration.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:30
The density of an unknown gas at 98°c and 740 mmhg is 2.50 g/l. what is the molar mass of the gas with work showed?
Answers: 1

Chemistry, 22.06.2019 17:30
Observation and experimentation have led many scientists to accept a theory about the origin of the universe. this theory is called the big bang theory. scientific evidence collected and observed by scientists around the world suggests that the universe is ever expanding from a hot and dense initial state. what makes this a scientific theory? (2 points)
Answers: 2

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1

Chemistry, 22.06.2019 22:30
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1
You know the right answer?
Suppose that some fescn2+ is added to the above solution to shift the equilibrium. when equilibrium...
Questions



Biology, 09.07.2021 19:20









Computers and Technology, 09.07.2021 19:20

Computers and Technology, 09.07.2021 19:20



Mathematics, 09.07.2021 19:20


Computers and Technology, 09.07.2021 19:20


![[FeSCN^{2+}] \rightleftharpoons [Fe^{3+}] + [SCN^{-}]](/tpl/images/0374/6366/acc98.png)
![[Fe^{3+}] = 8.17 \times 10^{-3}](/tpl/images/0374/6366/d6b48.png) M
M![[SCN^{-}] = 8.60 \times 10^{-3}](/tpl/images/0374/6366/bf96b.png) M
M![[FeSCN^{2+}] = 6.25 \times 10^{-2}](/tpl/images/0374/6366/063d9.png) M
M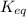 as follows.
as follows.![K_{eq} = \frac{[Fe^{3+}][SCN^{-}]}{[FeSCN^{2+}]}](/tpl/images/0374/6366/0a251.png)
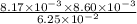

![[FeSCN^{2+}]](/tpl/images/0374/6366/797d4.png) will be calculated as follows.
will be calculated as follows.![11.24 \times 10^{-4} = \frac{8.12 \times 10^{-3} \times 7.84 \times 10^{-3}}{[FeSCN^{2+}]}](/tpl/images/0374/6366/07a9a.png)
![[FeSCN^{2+}] = 5.66 \times 10^{-2}](/tpl/images/0374/6366/abbb1.png) M
M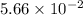 M.
M.

