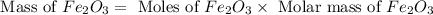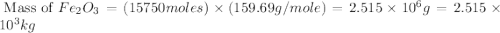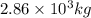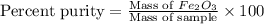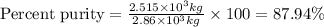One of the reactions that occurs in a blast furnace, in which iron ore is converted to cast iron, is fe2o3 + 3co → 2fe + 3co2 suppose that 1.79 × 103 kg of fe is obtained from a 2.86 × 103 kg sample of fe2o3. assuming that the reaction goes to completion, what is the percent purity of fe2o3 in the original sample?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Now consider the reaction when 45.0 g naoh have been added. what amount of naoh is this, and what amount of fecl3 can be consumed by it?
Answers: 3

Chemistry, 22.06.2019 09:30
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1

Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1

Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1
You know the right answer?
One of the reactions that occurs in a blast furnace, in which iron ore is converted to cast iron, is...
Questions

Mathematics, 09.11.2019 08:31


Biology, 09.11.2019 08:31

Social Studies, 09.11.2019 08:31



History, 09.11.2019 08:31


Mathematics, 09.11.2019 08:31

Mathematics, 09.11.2019 08:31







Mathematics, 09.11.2019 08:31

Mathematics, 09.11.2019 08:31

Mathematics, 09.11.2019 08:31

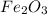 in the original sample is 87.94 %
in the original sample is 87.94 %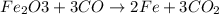
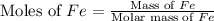
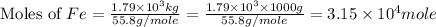
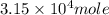 of Fe produced from
of Fe produced from 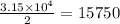 mole of
mole of