Chemistry, 15.11.2019 02:31 williejaroid123
Classify the possible combinations of signs for a reaction's δh and δs values by the resulting spontaneity
a. δh is positive and δs is negative
b. δh is positive and δs is positive
c. δh is negative and δs is positive
d. δh negative and δs is negative
for a, b, c and d find out which of following they are:
1. spontaneous as written at all temperatures
2. spontaneous in reverse at all temperatures
3. spontaneous as written above a certain temperature
4. spontaneous as written below a certain temperature
they can only have one answer but 2 can have the same answer

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 14:30
The length of a vector arrow represents its magnitude and the point represents its direction true or false apex
Answers: 3

Chemistry, 21.06.2019 19:00
Iknow the answer to 13 is b and 14 is d. i just need to know why the correct answers are correct
Answers: 1

Chemistry, 21.06.2019 22:30
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3

Chemistry, 21.06.2019 23:30
Problem #3 (ch. 1, problem 15)the ideal gas law provides one way to estimate the pressure exerted by a gas on a container. the law isí‘ťí‘ť=푛푛푛푛푛푛푉푉more accurate estimates can be made with the van der waals equationí‘ťí‘ť=푛푛푛푛푛푛푉푉â’푛푛푟푟â’푞푞푛푛2푉푉2where the term nb is a correction for the volume of the molecules and the term an2/v2is a correction for molecular attractions. the values of a and b depend on the type of gas. the gas constant is r, the absolutetemperature is t, the gas volume is v, and the number of moles of gas molecules is indicated by n. if n = 1 mol of an ideal gas were confined to a volume of v = 22.41 l at a temperature of 0â°c (273.2k), it would exert a pressure of 1 atm. in these units, r = 0.0826.for chlorine gas (cl2), a = 6.49 and b = 0.0562. compare the pressure estimates given by the ideal gas law and the van der waals equation for 1 mol of cl2 in 22.41 l at 273.2 k. what is the main cause of the difference in the two pressure estimates, the molecular volume or the molecular attractions?
Answers: 1
You know the right answer?
Classify the possible combinations of signs for a reaction's δh and δs values by the resulting spont...
Questions



Mathematics, 19.01.2021 01:50


Mathematics, 19.01.2021 01:50

Mathematics, 19.01.2021 01:50


English, 19.01.2021 01:50

Spanish, 19.01.2021 01:50

History, 19.01.2021 01:50


Arts, 19.01.2021 01:50





Mathematics, 19.01.2021 01:50


Mathematics, 19.01.2021 01:50

English, 19.01.2021 01:50

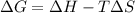
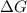 = Gibbs free energy
= Gibbs free energy 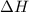 = enthalpy change
= enthalpy change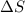 = entropy change
= entropy change 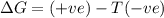
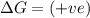
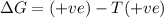
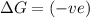 (at high temperature) (spontaneous)
(at high temperature) (spontaneous)