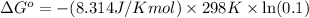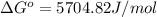Calculate δg°' for the reaction a + b < -> c + d at 25°c when the equilibrium concentrations are [a] = 10 um, [b] = 15 um, [c] = 3 um, and [d] = 5 um. r = 8.314 j/mol⋅k. give your answer to the nearest hundredths in j/mol, but only provide the numerical value (i. e. don't include units in your answer).

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:00
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3

Chemistry, 23.06.2019 01:00
Which of the following is a physical change? a.burning a piece of wood b.sawing a piece of wood in half c.rust forming on an iron fence d.a copper roof changing color from orange to green
Answers: 1

Chemistry, 23.06.2019 01:00
Which description best characterization the motion of particles in a solid
Answers: 1

Chemistry, 23.06.2019 03:30
Calculate the ph of a .10m nh4cl solution. the kb value for nh3 is 1.8×10^-5
Answers: 1
You know the right answer?
Calculate δg°' for the reaction a + b < -> c + d at 25°c when the equilibrium concentrations...
Questions



Mathematics, 28.09.2020 18:01

Biology, 28.09.2020 18:01


History, 28.09.2020 18:01


Advanced Placement (AP), 28.09.2020 18:01

Mathematics, 28.09.2020 18:01



Mathematics, 28.09.2020 18:01

Social Studies, 28.09.2020 18:01


Mathematics, 28.09.2020 18:01

Mathematics, 28.09.2020 18:01

English, 28.09.2020 18:01



Mathematics, 28.09.2020 18:01

![K=\frac{[C][D]}{[A][B]}](/tpl/images/0375/2516/80561.png)
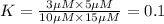
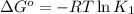
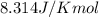
![25^oC=[273+25]K=298K](/tpl/images/0375/2516/0e82f.png)
 = equilibrium constant at 25°C = 0.1
= equilibrium constant at 25°C = 0.1