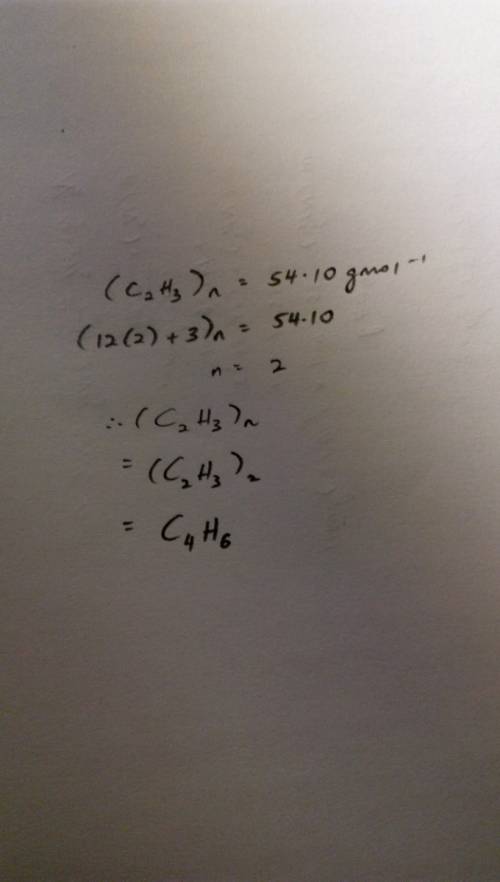Chemistry, 26.08.2019 12:30 kiarabermudez754
The empirical formula of a compound is determined to be c2h3, and its molecular mass is found to be 54.10 g/mol. determine the molecular formula of the compound, showing your work.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 16:50
What is the composition, in atom percent, of an alloy that consists of 4.5 wt% pb and 95.5 wt% sn? the atomic weights for pb and sn are 207.19 g/mol and 118.71 g/mol, respectively.(a) 2.6 at% pb and 97.4 at% sn(b) 7.6 at% pb and 92.4 at% sn(c)97.4 at% pb and 2.6 at% sn(d) 92.4 at% pb and 7.6 at% sn
Answers: 2

Chemistry, 21.06.2019 18:20
Which statement accurately describes the relationship between air pressure, air density, or altitude? as altitude increases, pressure increases.as altitude increases, air density increases.air pressure and density are lowest at sea level.denser air exerts more pressure than less dense air.
Answers: 2

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 2

Chemistry, 22.06.2019 03:00
Which best describes how johannes kepler developed his laws of planetary motion
Answers: 3
You know the right answer?
The empirical formula of a compound is determined to be c2h3, and its molecular mass is found to be...
Questions








Computers and Technology, 12.07.2019 03:10



Computers and Technology, 12.07.2019 03:10





Computers and Technology, 12.07.2019 03:10