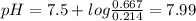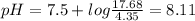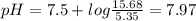Chemistry, 15.11.2019 06:31 cakecake15
A50.00-ml sample of bleach solution contains 0.214 m hclo and 0.667 m naclo. the ka of hypochlorous acid is 3.0 ✕ 10−8. find the ph of the solution. the solution is then divided in half. a) to one half of the original solution, 10.00 ml of 0.100 m naoh is added. what is the final ph of this solution? b) to the other half of the original solution, 1.00 ml of 0.100 m hcl is added. what is the final ph of this solution

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 19:00
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2

You know the right answer?
A50.00-ml sample of bleach solution contains 0.214 m hclo and 0.667 m naclo. the ka of hypochlorous...
Questions


World Languages, 28.02.2020 19:48






Mathematics, 28.02.2020 19:48


Mathematics, 28.02.2020 19:48



History, 28.02.2020 19:49

Mathematics, 28.02.2020 19:49






Mathematics, 28.02.2020 19:49

![pH=pKa+log\frac{[salt]}{[acid]}](/tpl/images/0375/5235/ec35f.png)