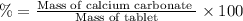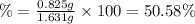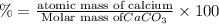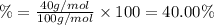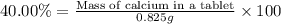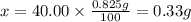Chemistry, 15.11.2019 07:31 harmonyfern5648
(a tablet containing calcium carbonate and fillers with a mass of 1.631 g was dissolved in hcl. after the fillers were filtered out, the hcl was neutralized by adding sodium carbonate. the resulting precipitate was pure calcium carbnonate (with the fillers removed). the solid calcium carbonate was collected on a watch glass that had a mass of 46.719 g when empty. after teh calcium carbonate had been allowed to dry, the mass of the watch glass plus product was found to be 47.544 g.
1. what is the mass of pure calcium carbonate product collected at the end of the experiment?
2. calculate the mass % calium carbonate in the tablet.
3. calculate the mass percent calcium in calcium carbonate. this calculation is theoretical and is independent of the data provided.
4. calculate the number of grams of calcium that were in the tablet.
(hint: this can be obtained by using the answers to question 1 and 3)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:00
What is driving behind plate tectonics (plate movment)? a) gravity only b) inertia c) convection and gravity d) the sun theres no option for science so i picked chemistry. plz
Answers: 2

Chemistry, 22.06.2019 12:00
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal.b. he is determining chemical properties that are sufficient to identify the metal.c. he is determining physical properties that are insufficient to identify the metal.d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3

Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2

Chemistry, 23.06.2019 00:30
An ice cube with a volume of 45.0ml and a density of 0.9000g/cm3 floats in a liquid with a density of 1.36g/ml. what volume of the cube is submerged in the liquid
Answers: 3
You know the right answer?
(a tablet containing calcium carbonate and fillers with a mass of 1.631 g was dissolved in hcl. afte...
Questions



History, 06.01.2021 18:30

Mathematics, 06.01.2021 18:30



Mathematics, 06.01.2021 18:30

Social Studies, 06.01.2021 18:30

Mathematics, 06.01.2021 18:30


Mathematics, 06.01.2021 18:30

Mathematics, 06.01.2021 18:30

Mathematics, 06.01.2021 18:30


Mathematics, 06.01.2021 18:30

English, 06.01.2021 18:30

Mathematics, 06.01.2021 18:30

Spanish, 06.01.2021 18:30


Mathematics, 06.01.2021 18:30