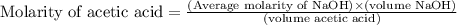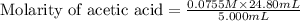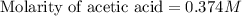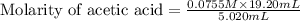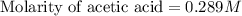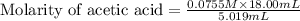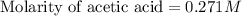Chemistry, 15.11.2019 18:31 beckers0115
Calculate and enter the molarity of your three acetic acid trials using the volume of standardized naoh solution required for each and the average molarity of the naoh solution from the standardization trials with khp. you should report 3 significant figures, e. g. 0.488 m.
entry # vol acetic acid(ml) vol na0h(ml) m acetic acid
#1: 5.000 24.80
#2: 5.020 19.20
#3: 5.019 18.00

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:30
This chart represents the melting point of several substance. what besy explains the high melting point of the salt?
Answers: 2

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

Chemistry, 22.06.2019 23:00
Condensation happens when water vapor cools. true or false? ?
Answers: 2
You know the right answer?
Calculate and enter the molarity of your three acetic acid trials using the volume of standardized n...
Questions


History, 03.07.2019 20:00





English, 03.07.2019 20:00

Mathematics, 03.07.2019 20:00

Spanish, 03.07.2019 20:00

History, 03.07.2019 20:00


Social Studies, 03.07.2019 20:00




English, 03.07.2019 20:00