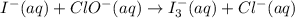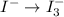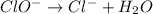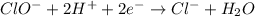Chemistry, 15.11.2019 19:31 truelove9288
Using the half reaction method balance to the redox equation in acidic solution: i-(aq) + clo-(aq) → i3-(aq) + cl-(aq) indicate the correct equation below. view available hint(s) using the half reaction method balance to the redox equation in acidic solution: i-(aq) + clo-(aq) → i3-(aq) + cl-(aq) indicate the correct equation below. 3i-(aq)+2h+(aq)+clo-(aq)→i3-(aq)+cl -(aq)+h2o(l) 3i-(aq)+2h+(aq)+clo-(aq)→i3-(aq)+cl -(aq)+h2o(l)+2e- i-(aq)+10h+(aq)+clo-(aq)→i3-(aq)+cl -(aq)+10h2o(l) i-(aq)+2h+(aq)+clo-(aq)→6i3-(aq)+cl -(aq)+h2o(l)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 16:00
Is this a scientific model? use complete sentences to explain why or why not. a graphic organizer showing the water cycle i need : ( asap i go it never mind
Answers: 2

Chemistry, 21.06.2019 22:30
Check the correct box to describe the periodic trends in electronegativity. electronegativity across a period: decreases. increases. electronegativity down a group: decreases. increases.
Answers: 2

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2
You know the right answer?
Using the half reaction method balance to the redox equation in acidic solution: i-(aq) + clo-(aq)...
Questions

Mathematics, 11.05.2021 03:10

History, 11.05.2021 03:10


Mathematics, 11.05.2021 03:10








Mathematics, 11.05.2021 03:10



Mathematics, 11.05.2021 03:10


Social Studies, 11.05.2021 03:10

Mathematics, 11.05.2021 03:10


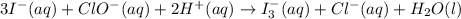
 at that side where the less number of hydrogen are present.
at that side where the less number of hydrogen are present.