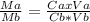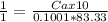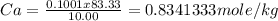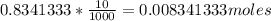Chemistry, 15.11.2019 19:31 jnsebastian2002
Vinegar is a dilute solution ethanoic acid in water (see the question above for the structure of ethanoic acid). in order to test the strength of a vinegar, a 10.00 gram sample was titrated with sodium hydroxide (0.1001 mole/kg of solution). the mass of the. burette before the titration is 131.44 g and upon reaching the endpoint the burette weighted 48.11 g. how many moles of acetic acid are in the sample?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3

Chemistry, 23.06.2019 01:00
Which statement best describes isomers? a. isomers are alcohols that have the same functional group. b. isomers have at least one carbon-carbon double bond. c. isomers have the same molecular formula but different structural properties.
Answers: 1

Chemistry, 23.06.2019 03:30
Mr. rose asked his student to draw a quadrilateral with four unequal sides. an example of this kind of quadrilateral
Answers: 1
You know the right answer?
Vinegar is a dilute solution ethanoic acid in water (see the question above for the structure of eth...
Questions


Computers and Technology, 10.04.2020 01:28


Mathematics, 10.04.2020 01:28


Mathematics, 10.04.2020 01:28

Physics, 10.04.2020 01:28

Mathematics, 10.04.2020 01:28

Mathematics, 10.04.2020 01:28


Mathematics, 10.04.2020 01:28


Biology, 10.04.2020 01:29



Mathematics, 10.04.2020 01:29

Biology, 10.04.2020 01:29

History, 10.04.2020 01:29

Chemistry, 10.04.2020 01:29

Advanced Placement (AP), 10.04.2020 01:29