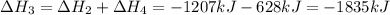Chemistry, 15.11.2019 19:31 makayyafreeman
Use the standard reaction enthalpies given below to determine δh°rxn for the following reaction: p4(g) + 10 cl2(g) → 4pcl5(s) δh°rxn = ? given: pcl5(s) → pcl3(g) + cl2(g) δh°rxn= +157 kj p4(g) + 6 cl2(g) → 4 pcl3(g) δh°rxn = -1207 kj

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1
You know the right answer?
Use the standard reaction enthalpies given below to determine δh°rxn for the following reaction: p4...
Questions

Mathematics, 16.10.2020 15:01

Health, 16.10.2020 15:01




Biology, 16.10.2020 15:01



Business, 16.10.2020 15:01



Mathematics, 16.10.2020 15:01

Chemistry, 16.10.2020 15:01

Mathematics, 16.10.2020 15:01


Mathematics, 16.10.2020 15:01

Advanced Placement (AP), 16.10.2020 15:01



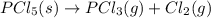
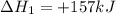 (1)
(1)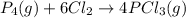
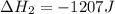 (2)
(2)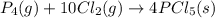
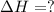 (3)
(3)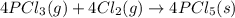
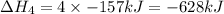 (4)
(4)