Chemistry, 15.11.2019 20:31 Mrblunt5613
Acetylene burns in air according to the following equation: c2h2(g) + 5 2 o2(g) → 2 co2(g) + h2o(g) δh o rxn = −1255.8 kj given δh o f of co2(g) = −393.5 kj/mol and δh o f of h2o(g) = −241.8 kj/mol, find δh o f of c2h2(g).

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:00
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1
You know the right answer?
Acetylene burns in air according to the following equation: c2h2(g) + 5 2 o2(g) → 2 co2(g) + h2o(g)...
Questions

Mathematics, 14.09.2020 15:01

English, 14.09.2020 15:01

Biology, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

Biology, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

History, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

Health, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

Spanish, 14.09.2020 15:01

Mathematics, 14.09.2020 15:01

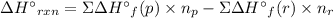
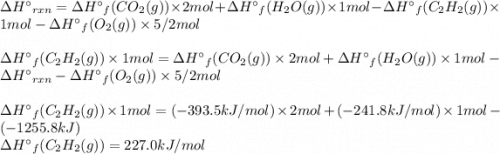
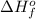 , of C₂H₂ is 227 kJ/mol
, of C₂H₂ is 227 kJ/mol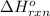 = −1255.8 kJ
= −1255.8 kJ