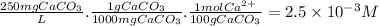The buffer solution is used to control the ph to insure that it does not become too high because excessively basic solutions could cause the corresponding hydroxides of hard metal ions (such as ca(oh)2 and mg(oh)2) to precipitate. using the calcium ion as a typical representative, just how high a ph do you think could be considered as "too high" for a solution with a hardness of about 250 ppm caco3? ksp for ca(oh)2 is 6.5 x 10-6.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1

Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2

Chemistry, 22.06.2019 23:30
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1
You know the right answer?
The buffer solution is used to control the ph to insure that it does not become too high because exc...
Questions











English, 07.04.2020 15:48

Mathematics, 07.04.2020 15:48

Social Studies, 07.04.2020 15:48

English, 07.04.2020 15:48

Mathematics, 07.04.2020 15:48





Computers and Technology, 07.04.2020 15:48