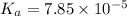Answers: 2
Another question on Chemistry

Chemistry, 23.06.2019 05:00
C=59(f−32)the equation above shows how temperature f, measured in degrees fahrenheit, relates to a temperature c, measured in degrees celsius. based on the equation, which of the following must be true? a temperature increase of 1 degree fahrenheit is equivalent to a temperature increase of 59 degree celsius.a temperature increase of 1 degree celsius is equivalent to a temperature increase of 1.8 degrees fahrenheit.a temperature increase of 59 degree fahrenheit is equivalent to a temperature increase of 1 degree celsius.a) i onlyb) ii onlyc) iii onlyd) i and ii only
Answers: 1

Chemistry, 23.06.2019 14:20
Compounds a and b react to form compounds c and d according to the equation: aa + bb → cc + dd. under which conditions will the rate law be given by the equation: rate = k[a]a[b]b? a. the reaction takes place in one step. b. the reaction is endothermic. c. the reaction is exothermic. d. the reaction involves more than one step.
Answers: 3

Chemistry, 23.06.2019 15:00
The coriolis effect influences neither wind speed nor wind direction wind speed both wind speed and wind direction wind direction
Answers: 1

You know the right answer?
1.50 g of a weak acid (molar mass 176) is dissolved in 50.0 ml of water, and the resultant solution...
Questions

Social Studies, 22.08.2019 03:30






Social Studies, 22.08.2019 03:30


Mathematics, 22.08.2019 03:30






History, 22.08.2019 03:30


Mathematics, 22.08.2019 03:30

Biology, 22.08.2019 03:30

Mathematics, 22.08.2019 03:30

Mathematics, 22.08.2019 03:30

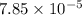 .
.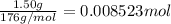
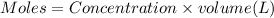
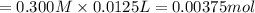
![pH=pK_a+\log(\frac{[salt]}{[acid]})](/tpl/images/0376/5102/e4eea.png)

![pH=pK_a+\log(\frac{[NaA]}{[HA]})](/tpl/images/0376/5102/83d67.png)
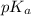 = ?
= ?![[HA]=\frac{0.008523 mol-0.00375}{0.0625 L}=\frac{0.004773 mol}{0.0625 L}](/tpl/images/0376/5102/6fe77.png)
![[NaA]=\frac{0.00375 mol}{0.0625 L}](/tpl/images/0376/5102/93c44.png)
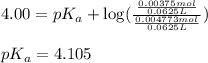
![4.105=-\log[K_a]](/tpl/images/0376/5102/b305f.png)