
In methane combustion, the following reaction pair is important: at 1500 k, the equilibrium constant kp has a value of 0.003691 based on a reference-state pressure of 1 atm (101,325 pa). derive an algebraic ex- pression for the forward rate coefficient kf . evaluate your expression for a temperature of 1500 k. give units.

Answers: 1
Another question on Chemistry



Chemistry, 21.06.2019 23:50
How does a scientist the government? a. the scientist tells people in society what to do. b. the scientist determines the policies that the government spends money on. c. the scientist provides unbiased information to the government. d. the scientist makes laws based on his or her research results.
Answers: 1

Chemistry, 22.06.2019 01:30
Reaction rate depends on how many molecules are coming into contact with each other with enough energy to react. increasing the temperature of the reactants will increase -
Answers: 3
You know the right answer?
In methane combustion, the following reaction pair is important: at 1500 k, the equilibrium constan...
Questions



Mathematics, 19.05.2021 20:30

Mathematics, 19.05.2021 20:30

German, 19.05.2021 20:30

Mathematics, 19.05.2021 20:30

Medicine, 19.05.2021 20:30

Mathematics, 19.05.2021 20:30

Mathematics, 19.05.2021 20:30

English, 19.05.2021 20:30



Mathematics, 19.05.2021 20:30



History, 19.05.2021 20:30



Mathematics, 19.05.2021 20:30

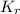 =
= 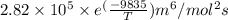
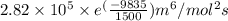
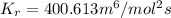
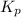 and
and 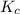 is as follows.
is as follows.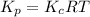
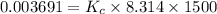
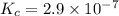
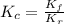
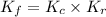
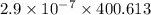
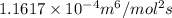
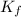 is
is 

