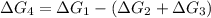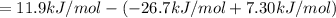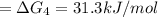Chemistry, 16.11.2019 00:31 dianasmygova
In nature, one common strategy to make thermodynamically unfavorable reactions proceed is to couple them chemically to reactions that are thermodynamically favorable. as long as the overall reaction is thermodynamically favorable, even the unfavorable reaction will proceed.
part a: consider these hypothetical chemical reactions:
a⇌b,δg= 11.9 kj/mol
b⇌c,δg= -26.7 kj/mol
c⇌d,δg= 7.30 kj/mol
what is the free energy, δg, for the overall reaction, a⇌d?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1

Chemistry, 21.06.2019 21:30
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10^-3 m and k for the dissociation is 1.86x10^-5. ch3cooh(aq)+h2o(> h3o^+(aq)+ch3coo^-(aq)
Answers: 2


Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2
You know the right answer?
In nature, one common strategy to make thermodynamically unfavorable reactions proceed is to couple...
Questions

Mathematics, 16.12.2020 14:00




Computers and Technology, 16.12.2020 14:00







English, 16.12.2020 14:00

History, 16.12.2020 14:00


Mathematics, 16.12.2020 14:00

Chemistry, 16.12.2020 14:00

English, 16.12.2020 14:00



Social Studies, 16.12.2020 14:00

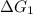 = 11.9 kJ/mol ...[1]
= 11.9 kJ/mol ...[1]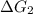 = -26.7 kJ/mol ...[2]
= -26.7 kJ/mol ...[2]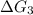 = 7.30 kJ/mol ...[3]
= 7.30 kJ/mol ...[3]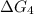 = ?...[4]
= ?...[4]