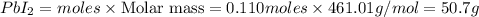Consider the balanced equation of k i ki reacting with p b ( n o 3 ) 2 pb(nox3)x2 to form a precipitate. 2 k i ( a q ) + p b ( n o 3 ) 2 ( a q ) ⟶ p b i 2 ( s ) + 2 k n o 3 ( a q ) 2ki(aq)+pb(nox3)x2(aq)⟶pbix2(s)+2kn ox3(aq) what mass of p b i 2 pbix2 can be formed by adding 0.528 l of a 0.417 m solution of k i ki to a solution of excess p b ( n o 3 ) 2 pb(nox3)x2?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:00
How did planetesmals form planets? a. they broke apart into smaller chunks.b. they collided and stuck together.c. they cooled and pulled ice together.d. they began to rotate.
Answers: 1

Chemistry, 23.06.2019 07:30
How many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride
Answers: 1

Chemistry, 23.06.2019 08:00
How many distinct monochlorinated products, including stereoisomers, can result when the alkane below is heated in the presence of cl2? 3 4 5 6 7?
Answers: 3

You know the right answer?
Consider the balanced equation of k i ki reacting with p b ( n o 3 ) 2 pb(nox3)x2 to form a precipit...
Questions






Arts, 02.12.2020 21:30





History, 02.12.2020 21:30


Physics, 02.12.2020 21:30



Mathematics, 02.12.2020 21:30

Biology, 02.12.2020 21:30

Mathematics, 02.12.2020 21:30


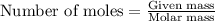
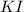
![\text{Number of moles}=molarity\times {\text {Volume in L]}=0.417M\times 0.528L=0.220moles](/tpl/images/0376/8666/592a1.png)
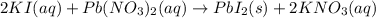
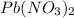 is in excess.
is in excess.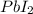
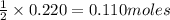 of
of