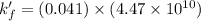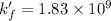A110.0 ml sample of 0.040 m ca2+ is titrated with 0.040 m edta at ph 9.00. the value of logkf for the ca2+−edta complex is 10.65 and the fraction of free edta in the y4− form, − , is 0.041 at ph 9.00. what is k′f , the conditional formation constant, for ca2+ at ph 9.00?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:20
What is the strongest intermolecular force between an nacl unit and an h2o molecule together in a solution? covalent bonding dipole-dipole force hydrogen bonding ion-dipole force
Answers: 1

Chemistry, 21.06.2019 23:00
Will mark brainliest26. which of these statements are true? (3 points)a. gases are compressibleb. gases fill their containers completelyc. the pressure of a gas is independent of the temperatured. gases have masse. gases exert pressuref. the pressure of a gas is dependent on the volumeg. gas pressure results from the collisions between gas particlesh. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 19:40
What causes different colors to appear in the sky? the absorption of light by air molecules the reflection of light by bodies of water the greenhouse effect in earth's atmosphere the scattering and reflection of light by dust particles
Answers: 2

Chemistry, 22.06.2019 20:30
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1
You know the right answer?
A110.0 ml sample of 0.040 m ca2+ is titrated with 0.040 m edta at ph 9.00. the value of logkf for th...
Questions

Mathematics, 25.09.2019 19:30


Mathematics, 25.09.2019 19:30

Biology, 25.09.2019 19:30




Mathematics, 25.09.2019 19:30

World Languages, 25.09.2019 19:30







History, 25.09.2019 19:30




English, 25.09.2019 19:30

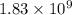
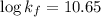
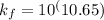
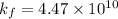
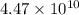
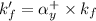
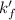 = conditional formation constant = ?
= conditional formation constant = ?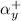 = activity coefficient at pH 9.00 = 0.041
= activity coefficient at pH 9.00 = 0.041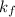 = formation constant =
= formation constant =