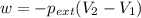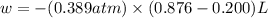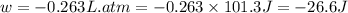Chemistry, 16.11.2019 05:31 INEEDHELP6845
Ascientist observed that a gas expanded from a volume of 0.200 l to a volume of 0.876 l. (a) what is the amount of work (in joules) performed in this process if the gas expanded at a constant pressure of 0.389 atm? w = j (b) if the temperature of the gas did not change during the expansion, calculate the change in internal energy of the gas, as well as the change in heat due to the expansion. δe = j q = j

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:30
The balanced chemical equation for this lab is: 3cucl2(aq) + 2al(s) 3cu(s) + 2alcl3(aq) if 10.5 g copper chloride react with 12.4 g aluminum, what is the limiting reactant?
Answers: 3

Chemistry, 21.06.2019 23:00
An electrons position cannot be known precisely only it's probability of being in a certain location can be known
Answers: 1


Chemistry, 23.06.2019 06:00
Robert leaves a chocolate bar in his car while attending school all day. when he goes to his car in the afternoon, the bat has changed into gooey liquid. what happened to the chocolate bar
Answers: 1
You know the right answer?
Ascientist observed that a gas expanded from a volume of 0.200 l to a volume of 0.876 l. (a) what is...
Questions

Health, 05.09.2021 09:30

Law, 05.09.2021 09:30

Health, 05.09.2021 09:30

History, 05.09.2021 09:30

Arts, 05.09.2021 09:30

Health, 05.09.2021 09:30

Computers and Technology, 05.09.2021 09:30

Mathematics, 05.09.2021 09:30


English, 05.09.2021 09:30


Mathematics, 05.09.2021 09:30

Health, 05.09.2021 09:30

Mathematics, 05.09.2021 09:30


English, 05.09.2021 09:30

Computers and Technology, 05.09.2021 09:30


English, 05.09.2021 09:30

Biology, 05.09.2021 09:30

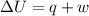
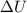 = internal energy
= internal energy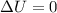
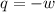
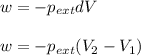
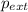 = external pressure = 0.389 atm
= external pressure = 0.389 atm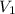 = initial volume of gas = 0.200 L
= initial volume of gas = 0.200 L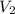 = final volume of gas = 0.876 L
= final volume of gas = 0.876 L