
Binary compounds of alkali metals and hydrogen react with water to liberate hydrogen gas. the hydrogen gas from the reaction of a sample of sodium hydride with an excess of water fills a volume of 0.475 l above the water. the temperature of the gas is 35 ∘c and the total pressure is 755 mmhg.
find the mass of h2 liberated and the mass of nah that reacted.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 22.06.2019 15:00
Why does a plastic bottle that is sealed at a high altitude change it’s shape when taken to lower altitude
Answers: 2

Chemistry, 22.06.2019 17:10
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1

Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2
You know the right answer?
Binary compounds of alkali metals and hydrogen react with water to liberate hydrogen gas. the hydrog...
Questions

Mathematics, 13.07.2019 12:00

Social Studies, 13.07.2019 12:00


Mathematics, 13.07.2019 12:00

Mathematics, 13.07.2019 12:00


History, 13.07.2019 12:00

Biology, 13.07.2019 12:00

Biology, 13.07.2019 12:00

Computers and Technology, 13.07.2019 12:00

Mathematics, 13.07.2019 12:00

Biology, 13.07.2019 12:00

History, 13.07.2019 12:00

History, 13.07.2019 12:00

Mathematics, 13.07.2019 12:00

Mathematics, 13.07.2019 12:00




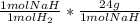 = 0.4480 g NaH
= 0.4480 g NaH

