
Chemistry, 16.11.2019 05:31 isabelle1670
The neutralization of h3po4 with koh is exothermic. h3po4(aq)+3koh(aq)⟶3h2o(l)+k3po4(aq )+173.2 kj if 60.0 ml of 0.200 m h3po4 is mixed with 60.0 ml of 0.600 m koh initially at 23.43 °c, predict the final temperature of the solution, assuming its density is 1.13 g/ml and its specific heat is 3.78 j/(g·°c). assume that the total volume is the sum of the individual volumes.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Will mark brainliest 26. which of these statements are true? (3 points) a. gases are compressible b. gases fill their containers completely c. the pressure of a gas is independent of the temperature d. gases have mass e. gases exert pressure f. the pressure of a gas is dependent on the volume g. gas pressure results from the collisions between gas particles h. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 03:00
Compare the valence electron configuration of the nobles gas elements seen here. what statement is correct?
Answers: 2

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 14:30
Which of the following is not one of the steps in the scientific method a. hypothesize b. summarize c. analyze d. familiarize
Answers: 3
You know the right answer?
The neutralization of h3po4 with koh is exothermic. h3po4(aq)+3koh(aq)⟶3h2o(l)+k3po4(aq )+173.2 kj i...
Questions


English, 10.03.2021 20:40

Mathematics, 10.03.2021 20:40


Mathematics, 10.03.2021 20:40

History, 10.03.2021 20:40


Mathematics, 10.03.2021 20:40


Geography, 10.03.2021 20:40


Mathematics, 10.03.2021 20:40

Computers and Technology, 10.03.2021 20:40



Mathematics, 10.03.2021 20:40

Mathematics, 10.03.2021 20:40



Mathematics, 10.03.2021 20:40

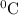
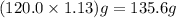
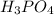 =
= 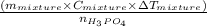
 represents change in temperature and n is number of moles
represents change in temperature and n is number of moles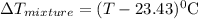
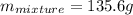 and
and 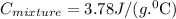
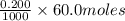 = 0.012 moles
= 0.012 moles![(173.2\times 10^{3})J=\frac{[(135.6g)\times (3.78J.g^{-1}.^{0}\textrm{C}^{-1})\times (T-23.43)^{0}\textrm{C}]}{0.012}](/tpl/images/0377/0998/64167.png)


