
**a piece of marble (assume it is pure caco3) reacts with 2.00l of 2.52 m hcl. after dissolution of the marble, a 10.00 ml sample of the resulting solution is withdrawn, added to some water, and titrated with 24.87 ml of 0.9987 m naoh. what must have been the mass of the piece of marble?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 21:20
One way in which the useful metal copper is produced is by dissolving the mineral azurite, which contains copper(ii) carbonate, in concentrated sulfuric acid. the sulfuric acid reacts with the copper(ii) carbonate to produce a blue solution of copper(ii) sulfate. scrap iron is then added to this solution, and pure copper metal precipitates out because of the following chemical reaction: (s) (aq) (s) (aq) suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. he adds powdered iron to a copper(ii) sulfate sample from the plant until no more copper will precipitate. he then washes, dries, and weighs the precipitate, and finds that it has a mass of .
Answers: 2

Chemistry, 22.06.2019 22:00
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3

Chemistry, 23.06.2019 01:20
How can parts of a solution be separated by chromatography?
Answers: 1
You know the right answer?
**a piece of marble (assume it is pure caco3) reacts with 2.00l of 2.52 m hcl. after dissolution of...
Questions


Biology, 15.12.2020 01:40


Mathematics, 15.12.2020 01:40




Mathematics, 15.12.2020 01:40




Mathematics, 15.12.2020 01:40


World Languages, 15.12.2020 01:40

Business, 15.12.2020 01:40

Mathematics, 15.12.2020 01:40


Mathematics, 15.12.2020 01:40


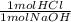 = 0.02483 mol HCl
= 0.02483 mol HCl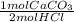 *
*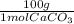 = 3.7 g marble
= 3.7 g marble

