

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:10
What approach is required to balance the objectives of sustainable development? balancing the objectives of sustainable development requires a(n) .
Answers: 3


Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1

Chemistry, 22.06.2019 19:30
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1
You know the right answer?
What would be the freezing point of a 1.7 mole aqueous ethylene glycol solution? the freezing point...
Questions






Biology, 13.11.2020 20:10



Mathematics, 13.11.2020 20:10



Mathematics, 13.11.2020 20:10






Mathematics, 13.11.2020 20:10

English, 13.11.2020 20:10

Mathematics, 13.11.2020 20:10

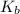 = 1.8 deg C/m
= 1.8 deg C/m
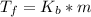
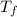 =1.8* 1.7
=1.8* 1.7


