
Acetylene reacts with oxygen to form carbon dioxide and water according to the following unbalanced reaction:
c2h2 (g) + o2 (g) → co2 (g) + h2o (g)
if we start with 38.7 g of acetylene and 13.7 g of oxygen, how many grams of the excess reactant are leftover after the reaction is complete?
report your answer to three significant figures.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 14:30
Which of the following is not one of the steps in the scientific method a. hypothesize b. summarize c. analyze d. familiarize
Answers: 3

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1
You know the right answer?
Acetylene reacts with oxygen to form carbon dioxide and water according to the following unbalanced...
Questions



Social Studies, 23.02.2021 19:00

Law, 23.02.2021 19:00



Mathematics, 23.02.2021 19:00







English, 23.02.2021 19:00


Mathematics, 23.02.2021 19:00

Physics, 23.02.2021 19:00



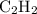 .
. 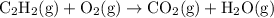
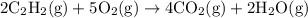
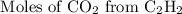
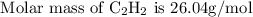
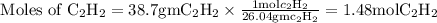
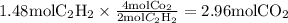
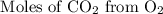 :
: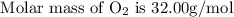
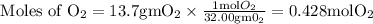
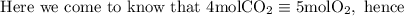
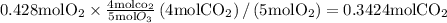
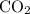 So
So 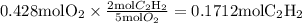
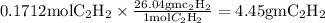
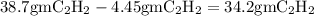 .
.

