
Achemist mixes 1.00 g cucl2 with an excess of (nh4)2hpo4 in dilute aqueous solution . he measures the evolution of 670 j of heat as the two substances react to give cu3(po4)2(s). compute the δh that would result from the reaction of 1.00 mo! cucl2 with an excess of (nh4)2hpo4.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 22:30
What relationship exists between an enzyme and a catalyst?
Answers: 1


Chemistry, 23.06.2019 07:30
Achemist at a pharmaceutical company is measuring equilibrium constants for reactions in which drug candidate molecules bind to a protein involved in cancer. the drug molecules bind the protein in a 1: 1 ratio to form a drug-protein complex. the protein concentration in aqueous solution at 25 ˚c is 1.74 x10-6 m . drug a is introduced into the protein solution at an initial concentration of 2.00 x10-6m. drug b is introduced into a separate, identical protein solution at an initial concentration of 2.00 x10-6m. at equilibrium, the drug a-protein solution has an a-protein complex concentration of 1.00 x10-6m, and the drug b solution has a b-protein complex concentration of 1.40 x10-6m.a. calculate the kc value for the a-protein binding reaction.b. calculate the kc value for the b-protein binding reaction.c. assuming that the drug that binds more strongly will be more effective, which drug is the better choice for further research?
Answers: 1
You know the right answer?
Achemist mixes 1.00 g cucl2 with an excess of (nh4)2hpo4 in dilute aqueous solution . he measures th...
Questions

Social Studies, 09.03.2021 09:30


Mathematics, 09.03.2021 09:30


Chemistry, 09.03.2021 09:30


History, 09.03.2021 09:30



Social Studies, 09.03.2021 09:30

History, 09.03.2021 09:30


English, 09.03.2021 09:30

History, 09.03.2021 09:30

History, 09.03.2021 09:30

Mathematics, 09.03.2021 09:30

Mathematics, 09.03.2021 09:30

Chemistry, 09.03.2021 09:30

Mathematics, 09.03.2021 09:30

Social Studies, 09.03.2021 09:30

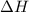 will be 90054 J
will be 90054 J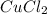 = 134.45 g/mol
= 134.45 g/mol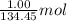 of
of 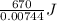 of heat or 90054 J of heat
of heat or 90054 J of heat

