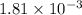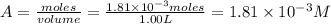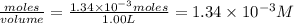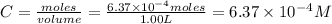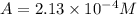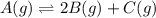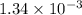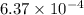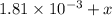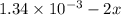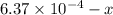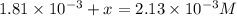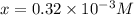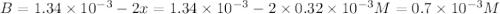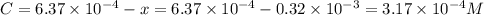Be sure to answer all parts. compound a decomposes according to the equation a(g) ⇌ 2 b(g) + c (g) a sealed 1.00−l container initially contains 1.81 × 10−3 mol of a(g), 1.34 × 10−3 mol of b(g), and 6.37 × 10−4 mol of c(g) at 100°c. at equilibrium, [a] is 2.13 × 10−3 m. find [b] and [c]. solve for the equilibrium concentrations of b and c. [b]eq × 10 m [c]eq × 10 m

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:10
Which statement describes both homogeneous mixtures and heterogeneous mixtures?
Answers: 1

Chemistry, 22.06.2019 11:00
When hydrochloric acid reacts with potassium hydroxide solution, the following reaction occurs. hcl (aq) + koh (aq) h2o (l) + kcl (aq) the reaction gives off heat energy, so it is an reaction.
Answers: 1

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3

Chemistry, 22.06.2019 17:30
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2
You know the right answer?
Be sure to answer all parts. compound a decomposes according to the equation a(g) ⇌ 2 b(g) + c (g) a...
Questions

English, 23.10.2021 23:10

Physics, 23.10.2021 23:10


History, 23.10.2021 23:10


Biology, 23.10.2021 23:10

Mathematics, 23.10.2021 23:10

Mathematics, 23.10.2021 23:10


Mathematics, 23.10.2021 23:10





History, 23.10.2021 23:20


English, 23.10.2021 23:20


Mathematics, 23.10.2021 23:20

Biology, 23.10.2021 23:20

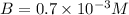
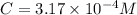
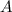 =
=