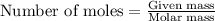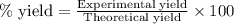Chemistry, 18.11.2019 19:31 puchie1225
A15.0 ml sample of a 1.92 m potassium sulfate solution is mixed with 14.9 ml of a 0.860 m barium nitrate solution and this precipitation reaction occurs: k2so4(aq) ba(no3)2(aq)→baso4(s) 2kno3(aq) the solid baso4 is collected, dried, and found to have a mass of 2.46 g . determine the limiting reactant, the theoretical yield, and the percent yield. part a determine the limiting reactant. express your answer as a chemical formula.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2

Chemistry, 23.06.2019 12:30
Choose one literary selection from this semester in which you think the setting has a great impact on the work. in a full paragraph name the work, describe the setting, and explain why it is so important to the overall story or poem.
Answers: 1

You know the right answer?
A15.0 ml sample of a 1.92 m potassium sulfate solution is mixed with 14.9 ml of a 0.860 m barium nit...
Questions

Mathematics, 17.04.2020 23:34

History, 17.04.2020 23:34







Physics, 17.04.2020 23:34








English, 17.04.2020 23:35



Social Studies, 17.04.2020 23:35

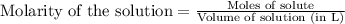 .....(1)
.....(1)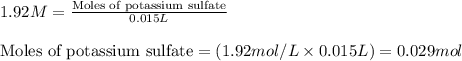

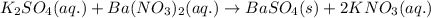
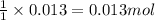 of potassium sulfate.
of potassium sulfate.