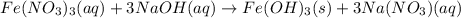Chemistry, 18.11.2019 19:31 meghan0123
When a solution of iron(iii) nitrate is mixed with a solution of sodium hydroxide, a rust colored precipitate forms. this precipitate is probably when a solution of iron(iii) nitrate is mixed with a solution of sodium hydroxide, a rust colored precipitate forms. this precipitate is probably a. iron (iii) nitrate. b. sodium hydroxide. c. iron (iii) hydroxide. d. sodium nitrate. e. none of the above

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Consider the nuclear equation below. 239 > x + 4 he 94 2 what is x? 1.235 cm 96 2.243 u 92 3.235 u 92 4.243 cm 96
Answers: 2

Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 22.06.2019 14:10
Precision can be defined as the o exact center of a data set. o reproducibility of a measured value. o correlation between two variables that are measured in a data set agreement between a measured value and an accepted value.
Answers: 2

Chemistry, 23.06.2019 01:00
If i had 2 m naoh solution, what does the 2 m stand for? 2 molar, but 2 of a solute in 1
Answers: 1
You know the right answer?
When a solution of iron(iii) nitrate is mixed with a solution of sodium hydroxide, a rust colored pr...
Questions

Biology, 18.02.2021 14:00


History, 18.02.2021 14:00

Mathematics, 18.02.2021 14:00

Mathematics, 18.02.2021 14:00


Mathematics, 18.02.2021 14:00



Mathematics, 18.02.2021 14:00

Physics, 18.02.2021 14:00

Mathematics, 18.02.2021 14:00


English, 18.02.2021 14:00

Mathematics, 18.02.2021 14:00

Mathematics, 18.02.2021 14:00

Mathematics, 18.02.2021 14:00



Mathematics, 18.02.2021 14:00