
Chemistry, 18.11.2019 20:31 noahdeem135
Industrial production of nitric acid, which is used in many products including fertilizers and explosives, approaches 10 billion kg per year worldwide. the first step in its production is the exothermic oxidation of ammonia, represented by the following equation. 4 nh3(g) + 5 o2(g) → 4 no(g) + 6 h2o(g) δh⁰rxn = −902.0 kj if this reaction is carried out using 7.056 ✕ 103 g nh3 as the limiting reactant, what is the change in enthalpy?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
If a bottle of olive oil contains 1.4 kg of olive oil, what is the volume, in milliliters ( ml ), of the olive oil?
Answers: 1

Chemistry, 22.06.2019 08:00
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1

Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1

Chemistry, 22.06.2019 15:20
Which description best characterizes the motion of particles in a solid?
Answers: 2
You know the right answer?
Industrial production of nitric acid, which is used in many products including fertilizers and explo...
Questions








Computers and Technology, 24.07.2019 11:20


Social Studies, 24.07.2019 11:20

Chemistry, 24.07.2019 11:20




Computers and Technology, 24.07.2019 11:20



Computers and Technology, 24.07.2019 11:20

Mathematics, 24.07.2019 11:20

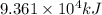
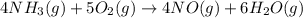
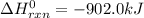
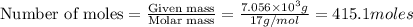
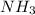 produces = 902.0 kJ of energy
produces = 902.0 kJ of energy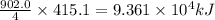 of energy
of energy

