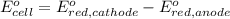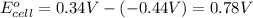In the activity, click on the e∘cell and keq quantities to observe how they are related. use this relation to calculate keq for the following redox reaction that occurs in an electrochemical cell having two electrodes: a cathode and an anode. the two half-reactions that occur in the cell are cu2+(aq)+2e−→cu(s) and fe(s)→fe2+(aq)+2e− the net reaction is cu2+(aq)+fe(s)→cu(s)+fe2+(aq) use the given standard reduction potentials in your calculation as appropriate.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
200. ml of 3.00 m nacl solution is diluted to a final volume of 500. ml. what is the molarity of the final solution?
Answers: 2

Chemistry, 21.06.2019 23:30
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

Chemistry, 22.06.2019 21:20
The organs inside the body and how they function together
Answers: 3
You know the right answer?
In the activity, click on the e∘cell and keq quantities to observe how they are related. use this re...
Questions




English, 26.06.2020 15:01

Mathematics, 26.06.2020 15:01


Physics, 26.06.2020 15:01





Mathematics, 26.06.2020 15:01



Health, 26.06.2020 15:01



Mathematics, 26.06.2020 15:01

Mathematics, 26.06.2020 15:01

Mathematics, 26.06.2020 15:01

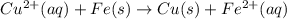
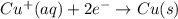
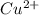 to Cu=
to Cu=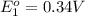
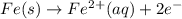
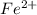 to Fe=
to Fe=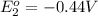
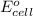 of the reaction, we use the equation:
of the reaction, we use the equation: