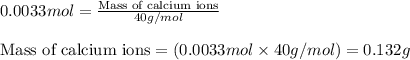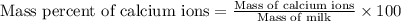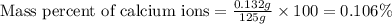Chemistry, 18.11.2019 22:31 kgonzalez200061
The amount of calcium present in milk can be determined through gravimetric analysis by adding oxalate to a sample and measuring the mass of calcium oxalate precipitated. what is the mass percent of calcium in milk if 0.429 g of calcium oxalate, cac2o4, forms in a 125-g sample of milk when excess aqueous sodium oxalate is added? na2c2o4 (aq) + ca2+ (aq) → cac2o4 (s) + 2 na+ (aq)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

Chemistry, 22.06.2019 10:50
How many grams of oxygen gas are contained in a 15 l sample at 1.02 atm and 28°c? show your work.
Answers: 1

You know the right answer?
The amount of calcium present in milk can be determined through gravimetric analysis by adding oxala...
Questions




Mathematics, 06.11.2020 20:00


Chemistry, 06.11.2020 20:00

Business, 06.11.2020 20:00



Mathematics, 06.11.2020 20:00

Mathematics, 06.11.2020 20:00


Mathematics, 06.11.2020 20:00

Mathematics, 06.11.2020 20:00


Mathematics, 06.11.2020 20:00

Mathematics, 06.11.2020 20:00

History, 06.11.2020 20:00

Mathematics, 06.11.2020 20:00

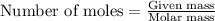 .....(1)
.....(1)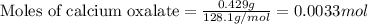
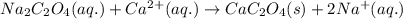
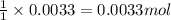 of calcium ions
of calcium ions