a. moles = molarity × volume.

All of the statements about molarity are correct except :
a. moles = molarity × volume.
b. the molarity of a diluted solution is less than the molarity of the original solution.
c. the abbreviation is m. volume = moles/molarity.
d. the interpretation of the symbol is "moles of solute per mole of solvent."

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:10
Harvey mixes two liquids. which observation of the new mixture most likely indicates a precipitate is forming?
Answers: 2

Chemistry, 21.06.2019 23:00
Will mark brainliest26. which of these statements are true? (3 points)a. gases are compressibleb. gases fill their containers completelyc. the pressure of a gas is independent of the temperatured. gases have masse. gases exert pressuref. the pressure of a gas is dependent on the volumeg. gas pressure results from the collisions between gas particlesh. gases have a definite volume and shape
Answers: 1

Chemistry, 21.06.2019 23:00
Achef makes salad dressing by mixing oil, vinegar, and spices, as shown. which type of matter is the salad dressing?
Answers: 1

Chemistry, 22.06.2019 00:00
How did planetesmals form planets? a. they broke apart into smaller chunks.b. they collided and stuck together.c. they cooled and pulled ice together.d. they began to rotate.
Answers: 1
You know the right answer?
All of the statements about molarity are correct except :
a. moles = molarity × volume.
a. moles = molarity × volume.
Questions

Mathematics, 28.10.2020 21:50



Mathematics, 28.10.2020 21:50


Mathematics, 28.10.2020 21:50

Mathematics, 28.10.2020 21:50

Social Studies, 28.10.2020 21:50

Mathematics, 28.10.2020 21:50


English, 28.10.2020 21:50

Mathematics, 28.10.2020 21:50

Mathematics, 28.10.2020 21:50

Mathematics, 28.10.2020 21:50




Mathematics, 28.10.2020 21:50

Health, 28.10.2020 21:50

Mathematics, 28.10.2020 21:50

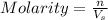
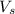 = volume of solution in L
= volume of solution in L

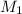 = molarity of stock solution
= molarity of stock solution 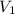 = volume of stock solution
= volume of stock solution 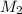 = molarity of diluted solution
= molarity of diluted solution 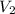 = volume of diluted solution
= volume of diluted solution


