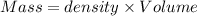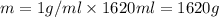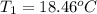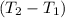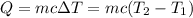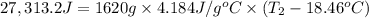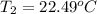Aquantity of 8.10 × 102 ml of 0.600 m hno3 is mixed with 8.10 × 102 ml of 0.300 m ba(oh)2 in a constant-pressure calorimeter of negligible heat capacity. the initial temperature of both solutions is the same at 18.46°c. the heat of neutralization when 1.00 mol of hno3 reacts with 0.500 mol ba(oh)2 is −56.2 kj/mol. assume that the densities and specific heats of the solution are the same as for water (1.00 g/ml and 4.184 j/g · °c, respectively). what is the final temperature of the solution?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 22.06.2019 17:00
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1

Chemistry, 22.06.2019 22:30
What relationship exists between an enzyme and a catalyst?
Answers: 1

Chemistry, 23.06.2019 00:50
What is the enthalpy of combustion (per mole) of c4h10 (g)? –2,657.5 kj/mol –5315.0 kj/mol –509.7 kj/mol –254.8 kj/mol
Answers: 1
You know the right answer?
Aquantity of 8.10 × 102 ml of 0.600 m hno3 is mixed with 8.10 × 102 ml of 0.300 m ba(oh)2 in a const...
Questions


Mathematics, 05.10.2019 22:40




History, 05.10.2019 22:40


Mathematics, 05.10.2019 22:40

Mathematics, 05.10.2019 22:40

Computers and Technology, 05.10.2019 22:40

Social Studies, 05.10.2019 22:40


History, 05.10.2019 22:40

Social Studies, 05.10.2019 22:40


History, 05.10.2019 22:40




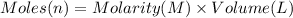
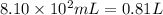
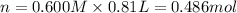
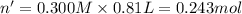
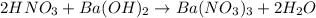
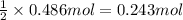 barium hydroxide.
barium hydroxide.