
The reaction system: pocl3(g) pocl(g) + cl2(g) is at equilibrium. which of the following statements describes the behavior of the system if pocl is added to the container? (a) the forward reaction will proceed to establish equilibrium.(b) the reverse reaction will proceed to establish equilibrium.(c) the partial pressures of pocl3 and pocl will remain steady while the partial pressure of chlorine increases.(d) the partial pressure of chlorine remains steady while the partial pressures of pocl3 and pocl increase.(e) the partial pressure of chlorine will increase while the partial pressure of pocl decreases.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 06:00
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3

Chemistry, 23.06.2019 01:00
Heat energy, carbon dioxide, and water are released through which process? a. photosynthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1
You know the right answer?
The reaction system: pocl3(g) pocl(g) + cl2(g) is at equilibrium. which of the following statements...
Questions

History, 01.10.2019 05:50

Physics, 01.10.2019 05:50



Mathematics, 01.10.2019 05:50

Mathematics, 01.10.2019 05:50

Mathematics, 01.10.2019 05:50


Mathematics, 01.10.2019 05:50



Physics, 01.10.2019 05:50




Health, 01.10.2019 05:50




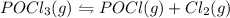
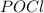 is added to the system
is added to the system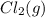 decreases and the partial pressure of
decreases and the partial pressure of 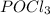 increases.
increases.


