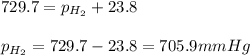Small amount wet of hydrogen gas (h2) can be prepared by the reaction of zinc with excess hydrochloric acid and trapping the gas produced in an inverted tube initially filled with water. if the total pressure of the gas in the collection tube is 729.7 mmhg at 25°c, what is the partial pressure of the hydrogen? the vapor pressure of water is 23.8 mmhg.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Write two balanced equations 1. dissolving of solid sodium hydroxide in water 2. the reaction of sodium hydroxide solution with hydrochloric acid
Answers: 1



Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3
You know the right answer?
Small amount wet of hydrogen gas (h2) can be prepared by the reaction of zinc with excess hydrochlor...
Questions

Mathematics, 23.09.2020 14:01


History, 23.09.2020 14:01


Mathematics, 23.09.2020 14:01


History, 23.09.2020 14:01

Mathematics, 23.09.2020 14:01

Mathematics, 23.09.2020 14:01

Mathematics, 23.09.2020 14:01




Physics, 23.09.2020 14:01

History, 23.09.2020 14:01





Mathematics, 23.09.2020 14:01

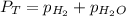
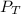 = 729.8 mmHg
= 729.8 mmHg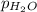 = 23.8 mmHg
= 23.8 mmHg