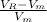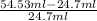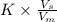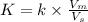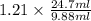Asample containing anthracene dissolved in methane was separated using gas chromatography on a column with mobile phase ) and stationary phase ) volumes of 24.7 ml and 9.88 ml, respectively. for this particular column, anthracene has a retention volume ) of 54.53 ml. calculate the retention factor and the partition coefficient (k) for anthracene in this separation.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 13:00
What is common about these molecules? a.their atoms are held together by covalent bonds. b.they are all made up of the same two atoms. c.their atoms are held together by ionic bonds. d.they are all made up of oxygen atoms only.
Answers: 3

Chemistry, 21.06.2019 20:50
Which real-world scenarios below represent physical and chemical changes? -running a car -exploding fireworks -mixing water and powdered drink mix -combining oil and vinegar to make salad dressing -taking aspirin for a headache -diluting bleach with water-digesting dinner-spreading peanut butter on bread
Answers: 2

Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 10:30
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2
You know the right answer?
Asample containing anthracene dissolved in methane was separated using gas chromatography on a colum...
Questions

Mathematics, 21.09.2021 22:40

Mathematics, 21.09.2021 22:40

Social Studies, 21.09.2021 22:40




Mathematics, 21.09.2021 22:40

Mathematics, 21.09.2021 22:40

Mathematics, 21.09.2021 22:40

Biology, 21.09.2021 22:40

Mathematics, 21.09.2021 22:40




Mathematics, 21.09.2021 22:40

Mathematics, 21.09.2021 22:40

Mathematics, 21.09.2021 22:40

History, 21.09.2021 22:40

Mathematics, 21.09.2021 22:40

Business, 21.09.2021 22:40

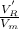
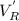 = adjusted retention volume =
= adjusted retention volume = 
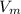 = hold up volume
= hold up volume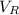 = total retention volume
= total retention volume