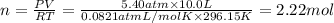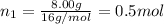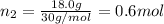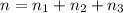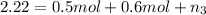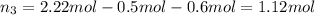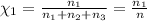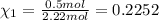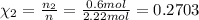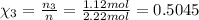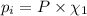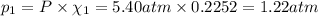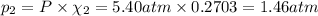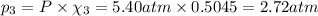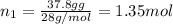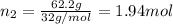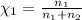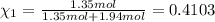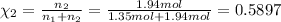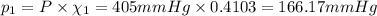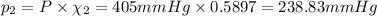Chemistry, 19.11.2019 01:31 kyliexhill
Part a: three gases (8.00 g of methane, ch_4, 18.0g of ethane, c_2h_6, and an unknown amount of propane, c_3h_8) were added to the same 10.0- l container. at 23.0 degrees c, the total pressure in the container is 5.40 atm. calculate the partial pressure of each gas in the container. part b: a gaseous mixture of o_2 and n_2 contains 37.8 % nitrogen by mass. what is the partial pressure of oxygen in the mixture if the total pressure is 405 mmhg?

Answers: 1
Another question on Chemistry

Chemistry, 20.06.2019 18:02
What is a spectator ion in the reaction between ba(no3)2(aq) and (nh4)3po4(aq)?
Answers: 2

Chemistry, 22.06.2019 07:30
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3
You know the right answer?
Part a: three gases (8.00 g of methane, ch_4, 18.0g of ethane, c_2h_6, and an unknown amount of pro...
Questions

Geography, 22.04.2021 03:00


Chemistry, 22.04.2021 03:00



Mathematics, 22.04.2021 03:00

Mathematics, 22.04.2021 03:00






Health, 22.04.2021 03:00


Chemistry, 22.04.2021 03:00

Mathematics, 22.04.2021 03:00


Chemistry, 22.04.2021 03:00

English, 22.04.2021 03:00

Mathematics, 22.04.2021 03:00