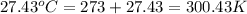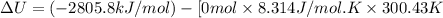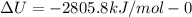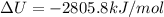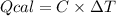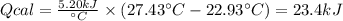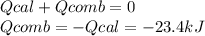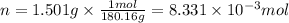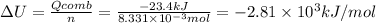Chemistry, 19.11.2019 02:31 Camill0310
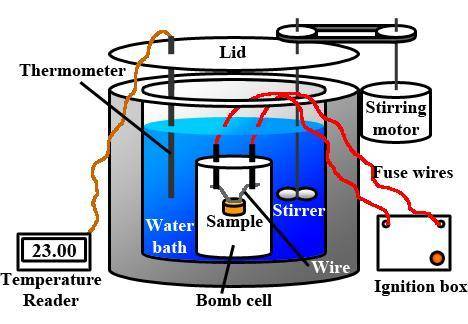
The combustion of 1.5011.501 g of fructose, c6h12o6(s)c6h12o6(s) , in a bomb calorimeter with a heat capacity of 5.205.20 kj/°c results in an increase in the temperature of the calorimeter and its contents from 22.9322.93 °c to 27.4327.43 °c. what is the internal energy change, δδu , for the combustion of 1.5011.501 g of fructose?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Layers of rock containing fossils, like the layers illustrated here, are most likely composed of rocks.
Answers: 2

Chemistry, 22.06.2019 13:00
One of the hopes for solving the world's energy problem is to make use of the fusion reaction 21h +31h --> 42he + 10n + energy how much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? the masses of the atoms and the neutrons are as follows: 21h = 2.0140 amu 31h = 3.01605 amu 42he = 4.002603 amu 10n = 1.008665 amu. the speed of light is 2.9979 x 108 m/s.
Answers: 1

Chemistry, 23.06.2019 00:20
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1

Chemistry, 23.06.2019 07:30
Which statement is actually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 2
You know the right answer?
The combustion of 1.5011.501 g of fructose, c6h12o6(s)c6h12o6(s) , in a bomb calorimeter with a heat...
Questions



Social Studies, 20.09.2020 07:01

History, 20.09.2020 07:01

Mathematics, 20.09.2020 07:01



History, 20.09.2020 07:01


Mathematics, 20.09.2020 07:01

Mathematics, 20.09.2020 07:01


English, 20.09.2020 07:01

History, 20.09.2020 07:01


Biology, 20.09.2020 07:01

English, 20.09.2020 07:01


Mathematics, 20.09.2020 07:01

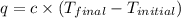
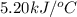
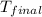 = final temperature =
= final temperature = 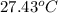
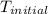 = initial temperature =
= initial temperature = 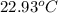
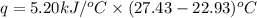
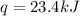
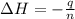
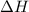 = enthalpy change = ?
= enthalpy change = ?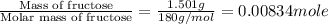
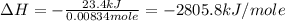
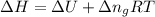
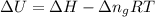

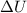 = change in internal energy = ?
= change in internal energy = ?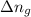 = change in moles = 0 (from the reaction)
= change in moles = 0 (from the reaction)