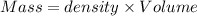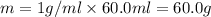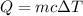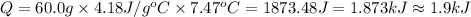Chemistry, 19.11.2019 02:31 Miloflippin9766
A50.0 ml of 0.88 m h2o2 and 10.0 ml of 0.50 m fe(no3)3 were combined and a temperature change of 7.47 was observed. the specific heat of water is 4.18 j/(g * ∘c) calculate the heat of reaction (in kilojoules). record your answer with the proper significant figures and include the correct sign if needed. assume the density and specific heat of the solution are the same as that of water. kj

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Check the correct box to describe the periodic trends in electronegativity. electronegativity across a period: decreases. increases. electronegativity down a group: decreases. increases.
Answers: 2

Chemistry, 22.06.2019 01:00
Which of the following is always a reactant in a combustion reaction? oxygen nitrogen hydrogen carbon
Answers: 1


Chemistry, 22.06.2019 12:00
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2
You know the right answer?
A50.0 ml of 0.88 m h2o2 and 10.0 ml of 0.50 m fe(no3)3 were combined and a temperature change of 7.4...
Questions



Law, 05.06.2020 04:57





Chemistry, 05.06.2020 04:57

History, 05.06.2020 04:57

History, 05.06.2020 04:57

Mathematics, 05.06.2020 04:57


Physics, 05.06.2020 04:57

Social Studies, 05.06.2020 04:57



Chemistry, 05.06.2020 04:57


Mathematics, 05.06.2020 04:57

Mathematics, 05.06.2020 04:57