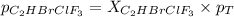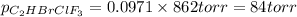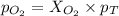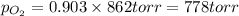Chemistry, 19.11.2019 02:31 marlenemedina247
Amixture of 15.0 g of the anesthetic halothane (c2hbrclf3 197.4 g/mol) and 22.6 g of oxygen gas has a total pressure of 862 torr. what are the partial pressures of each gas? a. phalothane = 778 torr, po2 = 84 torr b. phalothane = 162 torr, po2 = 700 torr c. phalothane = 84 torr, po2 = 778 torr d. phalothane = 155 torr, po2 = 707 torr e. phalothane = 707 torr, po2 = 155 torr.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 01:50
7. what temperature is need to just dissolve 50 g of nh4cl in 75 g of water? '
Answers: 1


Chemistry, 22.06.2019 16:00
Which factor is likely to impact the possible number of compounds ?
Answers: 1
You know the right answer?
Amixture of 15.0 g of the anesthetic halothane (c2hbrclf3 197.4 g/mol) and 22.6 g of oxygen gas has...
Questions

Mathematics, 18.03.2021 21:10







History, 18.03.2021 21:10

Social Studies, 18.03.2021 21:10





Biology, 18.03.2021 21:10





Mathematics, 18.03.2021 21:10

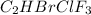 and
and 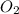 are, 84 torr and 778 torr respectively.
are, 84 torr and 778 torr respectively.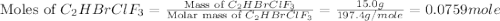
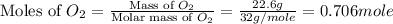
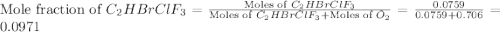
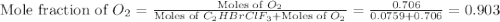
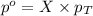
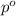 = partial pressure of gas
= partial pressure of gas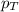 = total pressure of gas
= total pressure of gas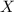 = mole fraction of gas
= mole fraction of gas