Chemistry, 19.11.2019 02:31 kaylallangari2145
Hydrogen iodide, hi, is formed in an equilibrium reaction when gaseous hydrogen and iodine gas are heated together. if 20.0 g of hydrogen and 20.0 g of iodine are heated, forming 10.0 g of hydrogen iodide, what mass of hydrogen remains unreacted? a. 10.0 g hydrogen remains b. 10.9 g hydrogen remains c. 15.0 g hydrogen remains d. 19.9 g hydrogen remains.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:00
Why do sodium and neon have vastly different chemical and physical properties despite having similar atomic masses?
Answers: 2

Chemistry, 22.06.2019 04:10
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2

Chemistry, 22.06.2019 08:30
How does the principle of electromagnetism explain the interaction between earth’s magnetic field and the solar wind?
Answers: 1

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1
You know the right answer?
Hydrogen iodide, hi, is formed in an equilibrium reaction when gaseous hydrogen and iodine gas are h...
Questions








SAT, 05.01.2022 02:20

SAT, 05.01.2022 02:20





English, 05.01.2022 02:20

Mathematics, 05.01.2022 02:30

Mathematics, 05.01.2022 02:30




History, 05.01.2022 02:30

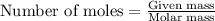
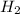
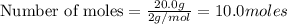
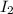
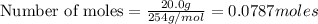
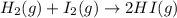
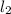 require=
require=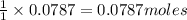 of
of